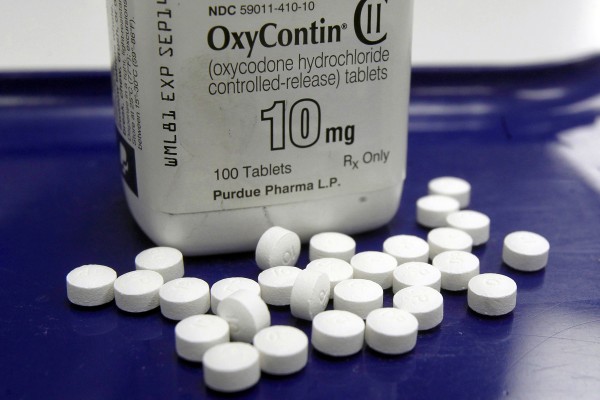
WASHINGTON (AP) — McKinsey & Company consulting firm has agreed to pay $650 million to settle a federal investigation into its work to help opioids manufacturer Purdue Pharma boost the sales of the the highly addictive drug OxyContin, according to court papers filed in Virginia on Friday.
As part of the deal with the U.S. Justice Department, McKinsey will avoid prosecution on criminal charges if it pays the sum and follows certain conditions for five years, including ceasing any work on the sale, marketing or promoting of controlled substances, according to the court papers.
A former McKinsey senior partner has also agreed to plead guilty to obstruction of justice for deleting documents from his laptop after he became aware of investigations into Purdue Pharma, according to the filings.
McKinsey said in a statement on Friday that it's “deeply sorry” for its work for Purdue Pharma.
“We should have appreciated the harm opioids were causing in our society and we should not have undertaken sales and marketing work for Purdue Pharma,” the company said. “This terrible public health crisis and our past work for opioid manufacturers will always be a source of profound regret for our firm.”
It's the latest effort by federal prosecutors to hold accountable companies officials say helped fuel the U.S. addiction and overdose crisis that has been linked to more than 80,000 deaths per year recently. For the past decade, most of the deaths have been attributed to illicit fentanyl, which is laced into many illegal drugs. Earlier in the epidemic, prescription pills were the primary cause of death.
Over the past eight years, drugmakers, wholesalers and pharmacies have agreed to about $50 billion worth of settlements with governments — and most of the money is required to be used to fight the crisis.
Purdue paid McKinsey more than $93 million over 15 years for several products, including how to improve revenue from OxyContin. Prosecutors say McKinsey “knew the risk and dangers” of OxyContin and knew that Purdue Pharma executives had previously pleaded guilty to crimes related to the promotion of the drug, but decided to work with the opioid manufacturer anyway.
During the work to “turbocharge” sales in 2013, McKinsey consultants accompanied Purdue sales representatives on visits to prescribers and pharmacies to gather information. In a note about one ride-along, a McKinsey consultant said one pharmacist had a gun “and was shaking; abuse is definitely a huge issue.” The company continued looking for ways to increase OxyContin sales, according to court papers.
One of the jobs for McKinsey, the papers said, was to identify which prescribers would generate the most additional prescriptions if Purdue salespeople focused on that. That resulted in prescriptions that “were not for a medically accepted indication, were unsafe, ineffective, and medically unnecessary, and that were often diverted for uses that lacked a legitimate medical purpose,” the filing said.
In 2014, McKinsey identified some small clinics that were writing more opioid prescriptions than entire hospital systems — and suggested they be targeted for more sales, the court filing said.
The company also tried to help Purdue get a say in shaping federal rules intended to ensure the benefits of addictive prescription drugs outweighed the risks. The government said in its new filings that that resulted in making high-dose OxyContin subject to the same oversight as lower-dose opioids and made training for prescribers voluntary rather than mandatory.
Since 2021, McKinsey has agreed to pay state and local governments about $765 million in settlements for its role in advising businesses on how to sell more of the powerful prescription painkillers amid a national opioid crisis.
The consulting firm also agreed last year to pay health care funds and insurance companies $78 million.
The U.S. has been in an addiction and overdose crisis for decades, linked to more than 80,000 deaths in recent years. For the past decade, most of the deaths have been attributed to illicit fentanyl, which is laced into many illegal drugs. Earlier in the epidemic, prescription pills were the primary cause of death.
Some advocates say the crisis was touched off when Purdue Pharma’s OxyContin hit the market in 1996.
Three Purdue executives pleaded guilty to misbranding charges in 2007 and the company agreed to pay a fine. The company pleaded guilty to criminal charges in 2020 and agreed to $8.3 billion in penalties and forfeitures — most of which will be waived as long as it executes a settlement through bankruptcy court that is still in the works.
McKinsey documents made public over the years describe Purdue using the consulting firm to help “turbocharge” opioid sales in 2013, as blowback against the opioid crisis meant that the company’s drugs were being prescribed less.
___
Mulvihill reported from Philadelphia.

http://accesswdun.com/article/2024/12/1276942
